Neonatal respiratory failure remains a primary cause of admission to neonatal intensive care units (NICUs) worldwide. Premature birth, affecting an estimated 15 million neonates annually globally, is strongly correlated with pulmonary immaturity and an elevated risk of respiratory complications (1). In such cases, respiratory support is frequently indispensable for reducing morbidity and improving survival outcomes.
Common causes of neonatal respiratory insufficiency include respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), pneumonia, and apnea of prematurity. Without timely intervention, these conditions can lead to rapid clinical deterioration and significant long-term sequelae, such as bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), or mortality.
The physiological immaturity of the neonatal lung significantly contributes to the high incidence of respiratory complications in preterm and term neonates with perinatal distress. In premature infants, incomplete alveolarization and insufficient surfactant production often lead to alveolar collapse.
These factors, coupled with a compliant chest wall, underdeveloped respiratory musculature, and increased oxygen demands, create a scenario where neonates are prone to atelectasis, hypoventilation, and respiratory failure (2). Consequently, mechanical or non-invasive ventilatory support is frequently initiated shortly after birth to stabilize gas exchange and reduce the work of breathing.
Over the past few decades, the field of neonatal respiratory support has evolved dramatically. Mechanical ventilation, once the cornerstone of neonatal respiratory care, has increasingly been supplanted by lung-protective strategies designed to minimize the risks of ventilator-induced lung injury (VILI).
Innovations such as volume-targeted ventilation (VTV), high-frequency oscillatory ventilation (HFOV), and neurally adjusted ventilatory assist (NAVA) have significantly improved synchronization and pressure control. Parallel advances in monitoring technology, including electrical impedance tomography (EIT) and artificial intelligence (AI)-based ventilator analytics, now offer real-time, individualized feedback for optimized care (3).
This review aims to provide a comprehensive overview of current strategies and innovations in neonatal respiratory support. It covers the physiological basis for respiratory assistance in neonates, details conventional and emerging ventilatory approaches, and explores the implications of novel technologies.
Additionally, it discusses the persistent challenges encountered in low-resource settings and outlines future directions for research and clinical practice. Through this review, we seek to inform clinicians, researchers, and policymakers about the current state and future potential of neonatal respiratory care worldwide.
Physiology and Pathophysiology in Neonatal Respiratory
The transition from intrauterine to extrauterine life presents substantial respiratory challenges for neonates, particularly those born prematurely. In utero, gas exchange occurs via the placenta, with the fetal lungs remaining fluid-filled and relatively inactive.
At birth, this abruptly changes, necessitating rapid clearance of lung fluid, the onset of spontaneous breathing, and functional pulmonary circulation. The success of this transition is highly dependent on lung maturity, surfactant availability, and coordinated cardiorespiratory function.
Lung development progresses through several distinct stages: embryonic, pseudoglandular, canalicular, saccular, and alveolar. Alveolarization—the formation of functional gas-exchange units—begins around 36 weeks of gestation and continues postnatally.
In preterm infants, especially those born before 32 weeks, alveolar and capillary networks are insufficiently developed to support efficient gas exchange (2). This underdevelopment contributes to poor lung compliance, increased airway resistance, and ineffective ventilation.
One of the most critical deficits in preterm lung function is the lack of surfactant, a lipoprotein complex that reduces surface tension and prevents alveolar collapse. Surfactant deficiency leads to decreased lung compliance, atelectasis, and impaired oxygenation—the hallmark features of Respiratory Distress Syndrome (RDS).
Additionally, the neonatal chest wall is highly compliant, offering little resistance to inward recoil, and the diaphragm is relatively underpowered, further limiting ventilation efficiency.
Several pathophysiological conditions arise from these anatomical and functional limitations. Transient Tachypnea of the Newborn (TTN) is common in term and near-term infants and is caused by delayed absorption of fetal lung fluid.
In contrast, apnea of prematurity results from the immaturity of brainstem respiratory centers, leading to episodic cessation of breathing. While the underlying mechanisms differ for these conditions, both may necessitate some form of respiratory assistance, whether invasive or non-invasive.
In addition to pulmonary immaturity, hemodynamic and systemic challenges—such as persistent pulmonary hypertension of the newborn (PPHN), patent ductus arteriosus (PDA), or sepsis—can compound respiratory compromise. Hypoxemia and acidosis further increase pulmonary vascular resistance, worsening oxygenation and potentially causing right-to-left shunting.
Collectively, these factors explain why many neonates, especially those born prematurely or with perinatal complications, require respiratory support. Understanding the unique physiology and vulnerabilities of the neonatal lung is essential for selecting the appropriate support strategy and optimizing outcomes.
| Condition | Gestational Age Affected | Pathophysiology | Clinical Features | CXR Findings | Initial Management | Need for Respiratory Support |
| Respiratory Distress Syndrome (RDS) | <32 weeks (mostly preterm) | Surfactant deficiency → alveolar collapse → poor gas exchange | Tachypnea, grunting, nasal flaring, cyanosis | Ground-glass opacities, air bronchograms | Surfactant, CPAP or MV, oxygen | High – often needs MV and surfactant |
| Transient Tachypnea of the Newborn (TTN) | Term or near-term | Delayed lung fluid clearance → mild pulmonary edema | Tachypnea shortly after birth, no distress signs | Prominent vascular markings, fluid in fissures | Observation, oxygen if needed | Low – usually resolves within 48–72 hrs |
| Apnea of Prematurity | <34 weeks | Immature central respiratory control → intermittent apnea | Episodes of apnea with bradycardia/desaturation | Often normal | Caffeine, CPAP in moderate/severe cases | Variable – often needs nasal CPAP |
| Meconium Aspiration Syndrome (MAS) | Term or post-term | Airway obstruction + inflammation → V/Q mismatch, hypoxemia | Respiratory distress, barrel chest, coarse crackles | Patchy infiltrates, hyperinflation | Oxygen, CPAP or MV, sometimes surfactant | Moderate – depends on severity |
| Persistent Pulmonary Hypertension (PPHN) | Term, near-term | Failure of normal pulmonary vasodilation → right-to-left shunting | Cyanosis unresponsive to O₂, loud second heart sound | Normal or variable | Oxygen, iNO, MV, sedation | High – may require HFOV or ECMO |
| Bronchopulmonary Dysplasia (BPD) | Very preterm, typically evolving | Chronic lung injury from prolonged O₂/MV exposure | Ongoing oxygen need >28 days, wheezing, poor growth | Hyperinflation, fibrosis, cystic changes | Diuretics, bronchodilators, O₂ | Chronic support — often home O₂ or ventilation |
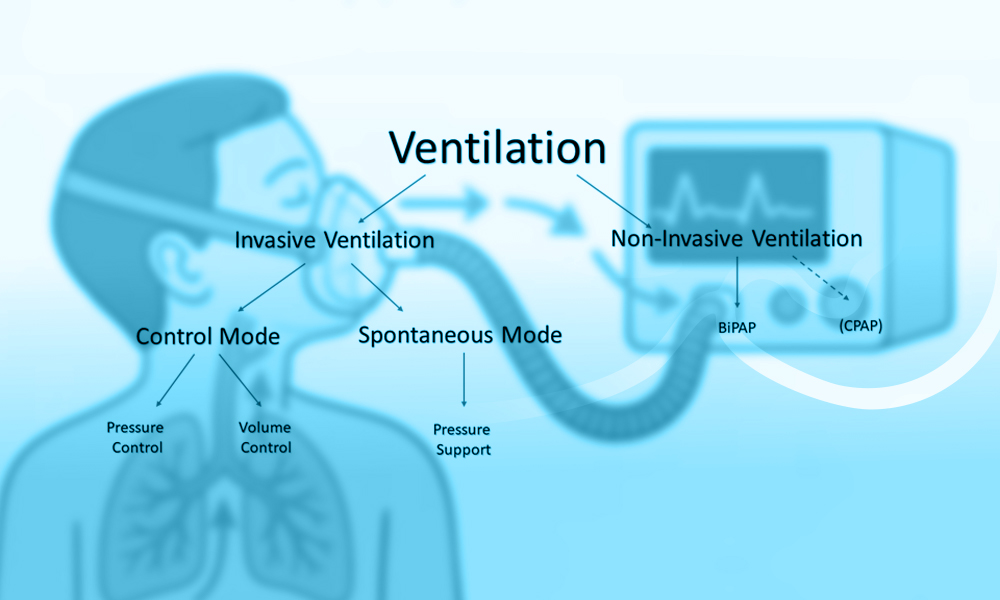
Invasive Ventilatory Support
Invasive mechanical ventilation (IMV) is a critical intervention for neonates with life-threatening respiratory failure, often due to surfactant deficiency, structural lung immaturity, or systemic complications such as sepsis or persistent pulmonary hypertension.
While IMV can be life-saving, it is associated with significant risks, necessitating careful selection of ventilatory modes, parameters, and weaning strategies to optimize outcomes and reduce harm (4, 5).
Invasive Ventilation Modes and Strategies
Several ventilatory modes are used in neonatal intensive care units (NICUs), each tailored to specific clinical needs:
- Assist-Control Ventilation (A/C): Delivers preset breaths, which can be initiated by the neonate or the ventilator. While maintaining adequate minute ventilation, this mode carries an increased risk of asynchrony and volutrauma (lung injury caused by excessive tidal volumes).
- Synchronized Intermittent Mandatory Ventilation (SIMV): Synchronizes mandatory breaths with spontaneous respiratory efforts, improving patient comfort and reducing the risk of hyperventilation.
- Volume-Targeted Ventilation (VTV): Automatically adjusts pressure to deliver consistent tidal volumes. Studies indicate that VTV reduces the incidence of bronchopulmonary dysplasia (BPD), hypocarbia, and intraventricular hemorrhage compared to pressure-limited modes (6, 7).
- High-Frequency Oscillatory Ventilation (HFOV): Utilizes rapid oscillations at small tidal volumes, thereby maintaining lung recruitment while minimizing barotrauma. Newer strategies now emphasize volume targeting during HFOV, leading to better control of CO₂ and improved outcomes (7, 8).
Lung-Protective Strategies
Modern neonatal ventilation prioritizes lung protection by minimizing injury from overdistension and repetitive collapse-reopening:
- Permissive Hypercapnia: This strategy tolerates moderate hypercapnia to reduce ventilation pressures and associated lung injury.
- Open Lung Strategy: This approach incorporates lung recruitment maneuvers and optimal PEEP (Positive End-Expiratory Pressure) titration to prevent atelectasis and minimize dynamic strain (9).
- Volume Guarantee Modes: Integrated into many modern ventilators, these modes ensure the delivery of targeted volumes during each breath, regardless of compliance changes.
Innovations such as artificial intelligence (AI)-guided pressure titration and real-time electrical impedance tomography (EIT) are increasingly integrated to personalize lung protection strategies (2, 3).
Risks and Adverse Outcomes
Invasive mechanical ventilation (IMV) is associated with several short- and long-term complications, including:
- Ventilator-Induced Lung Injury (VILI): This encompasses barotrauma, volutrauma, atelectrauma, and biotrauma, all of which are primary contributors to bronchopulmonary dysplasia (BPD).
- Ventilator-Associated Pneumonia (VAP): A significant nosocomial infection risk that can prolong NICU stay and worsen outcomes.
- Laryngeal and Airway Injury: Prolonged intubation can lead to subglottic stenosis and tracheomalacia, particularly in very low birth weight (VLBW) infants.
- Chronic Lung Disease: Up to 50% of preterm infants requiring prolonged IMV develop BPD, with lasting implications for pulmonary and neurodevelopment (10).
Extubation and Weaning in Neonates
The transition from invasive mechanical ventilation (IMV) to spontaneous breathing is a critical juncture in neonatal respiratory care. Premature or failed extubation can lead to increased morbidity, prolonged hospital stays, and a higher risk of complications such as ventilator-associated pneumonia (VAP), airway trauma, and bronchopulmonary dysplasia (BPD) (11). Therefore, the timing and strategy for weaning and extubation must be both evidence-based and individualized.
Physiological Readiness for Extubation
Successful extubation depends on the maturity and coordination of respiratory control, adequate gas exchange, and respiratory muscle endurance. Clinicians assess multiple physiological parameters before attempting extubation, including:
- Adequate Spontaneous Respiratory Drive: Demonstrated by consistent respiratory effort with an acceptable respiratory rate.
- Stable Gas Exchange: Pre-extubation arterial blood gases should show a pH > 7.25, PaCO₂ < 60 mmHg (for most neonates), and SpO₂ in the target range on an FiO₂ < 0.4.
- Minimal Ventilator Support: Indicated by peak inspiratory pressure (PIP) < 20 cmH₂O, PEEP ≤ 5 cmH₂O, and mean airway pressure (MAP) within safe limits.
- Hemodynamic Stability: Absence of significant cardiovascular instability, apnea, or bradycardia episodes.
Premature infants are especially vulnerable to extubation failure due to underdeveloped respiratory musculature, immature central respiratory control, and a highly compliant chest wall (12).
Clinical Predictors and Tools
While there is no universally accepted extubation readiness index, several approaches have shown promise:
- Spontaneous Breathing Trials (SBTs): Typically lasting 3–5 minutes, SBTs assess the neonate’s ability to breathe spontaneously with minimal pressure support. Success in SBTs correlates strongly with extubation success in older infants but has mixed predictive value in preterm infants (13).
- Extubation Readiness Scores (ERS): Composite tools incorporating variables like gestational age, respiratory pattern, minute ventilation, and neurological status are under investigation to standardize assessments (3).
- Diaphragmatic Ultrasound and EIT: Novel non-invasive imaging modalities such as diaphragmatic excursion measurement and electrical impedance tomography (EIT) can aid in assessing respiratory effort and lung aeration prior to extubation (2).
Weaning Strategies
Weaning protocols can be gradual or abrupt, depending on the patient’s underlying condition, lung maturity, and ventilator settings. Common strategies include:
- Stepwise Reduction: This involves progressively decreasing ventilator support parameters (e.g., Peak Inspiratory Pressure [PIP], respiratory rate, FiO₂) while continuously monitoring for signs of distress or desaturation.
- Mode Transitioning: This strategy involves transitioning from synchronized intermittent mandatory ventilation (SIMV) or assist-control (A/C) to pressure support ventilation (PSV) or continuous positive airway pressure (CPAP) before extubation.
- Volume Guarantee Titration: Ensuring the delivery of a minimum tidal volume, even during low spontaneous effort, helps prevent underventilation before extubation.
Extubation Failure and Its Implications
Extubation failure—defined as the need for reintubation within 48–72 hours—occurs in up to 30–40% of extremely preterm infants. Risk factors include:
- Birth weight < 1000g
- Gestational age < 28 weeks
- History of sepsis or intraventricular hemorrhage (IVH)
- High FiO₂ (> 0.5) at the time of extubation
- Poor weight gain or neuromuscular tone
The consequences of failed extubation are significant: repeated intubation increases the risk of vocal cord injury, subglottic stenosis, and worsens lung inflammation, potentially exacerbating bronchopulmonary dysplasia (BPD) (9, 11).
To mitigate this risk, post-extubation support strategies include the early use of non-invasive ventilation (e.g., nasal continuous positive airway pressure [nCPAP], bilevel positive airway pressure [BiPAP], high-flow nasal cannula [HFNC]) and pharmacologic agents like caffeine citrate, which has been shown to reduce apnea and improve extubation outcomes in preterm infants (10).
Non-Invasive Ventilation Strategies in Neonatal Respiratory
Non-invasive ventilation (NIV) plays a pivotal role in neonatal respiratory support, particularly in preterm infants, by providing respiratory assistance while avoiding the risks associated with endotracheal intubation. The primary goal of NIV is to maintain adequate gas exchange, reduce the work of breathing, and prevent lung injury associated with invasive ventilation. Over the past two decades, this field has evolved significantly, offering a range of NIV modalities with improved patient outcomes.
Modes of Non-Invasive Ventilation
Nasal Continuous Positive Airway Pressure (nCPAP) is the most widely used non-invasive technique in neonatal units. It operates by maintaining a constant distending pressure in the airways, thereby preventing alveolar collapse, improving functional residual capacity, and decreasing the work of breathing.
NCPAP is particularly effective in the early management of Respiratory Distress Syndrome (RDS) and has been shown to reduce the need for mechanical ventilation and the risk of bronchopulmonary dysplasia (14).
Nasal Intermittent Positive Pressure Ventilation (NIPPV) and its variant, Bilevel Positive Airway Pressure (BiPAP), deliver intermittent pressure boosts over a CPAP baseline. NIPPV improves minute ventilation, augments tidal volumes, and is especially effective during weaning or post-extubation. Studies indicate that NIPPV reduces extubation failure compared to nCPAP, although its superiority in primary respiratory support is less conclusive (15, 16).
Heated Humidified High-Flow Nasal Cannula (HHHFNC) delivers warmed, humidified air-oxygen blends at flow rates sufficient to wash out nasopharyngeal dead space and provide a low level of positive pressure. Its simplicity, comfort, and ease of use have led to its widespread adoption. However, HHHFNC may provide insufficient support in infants with moderate to severe RDS, particularly those younger than 28 weeks of gestation (17).
Non-invasive Neurally Adjusted Ventilatory Assist (NAVA) utilizes diaphragmatic electromyographic signals to trigger ventilator assistance, offering highly synchronized support. While promising, this modality is currently limited to specialized centers and remains under evaluation in large-scale clinical trials (18).
Interfaces and Delivery Systems
The effectiveness of non-invasive ventilation (NIV) is profoundly influenced by the interface used. Poorly fitted or inappropriate interfaces can lead to pressure leak, ineffective ventilation, or skin injury. A range of nasal interfaces is available, each with unique advantages and trade-offs:
| Interface Type | Pressure Delivery | Comfort/Tolerability | Risk of Nasal Trauma | Leak Management | Common Use |
| Short Binasal Prongs (e.g., Hudson) | Reliable, low resistance | Moderate | High (esp. septal) | Good fit required | CPAP, NIPPV |
| Nasal Masks | Broad surface area | Moderate–High | Moderate | Lower risk of leak | CPAP, NIPPV |
| RAM Cannula | Variable pressures | High | Low–Moderate | Prone to leak | Low-level CPAP |
| Nasopharyngeal Tube | Moderate, stable | Low | High | Minimal leak | CPAP (esp. in LMICs) |
There is no definitive consensus on the most effective interface; however, rotating between nasal prongs and masks is widely recommended to prevent skin breakdown (19, 20).
Failure Criteria and Escalation Indicators
Recognizing non-invasive ventilation (NIV) failure early is essential to avoid delays in mechanical ventilation, which are associated with higher morbidity. Failure criteria include:
- Arterial pH < 7.25 with PaCO₂ > 65 mmHg
- Sustained FiO₂ > 0.4 to maintain SpO₂ targets
- Recurrent apnea (>6 episodes/hour) or severe bradycardia
- Respiratory muscle fatigue and signs of distress
- Hemodynamic instability
Adjuncts such as rescue surfactant therapy—administered via the INSURE (Intubation, Surfactant, Extubation) or LISA (Less Invasive Surfactant Administration) methods—can help reduce the likelihood of NIV failure and progression to mechanical ventilation (17, 21).
Clinical Outcomes and Ongoing Controversies
Non-invasive ventilation (NIV) has been instrumental in reducing rates of intubation, bronchopulmonary dysplasia (BPD), and ventilator-associated complications. However, several debates remain unresolved:
- NIPPV vs. CPAP: While evidence supports NIPPV as superior for reducing extubation failure, its effectiveness in avoiding initial intubation is not uniformly conclusive (15).
- HFNC as Primary Support: Although user-friendly and well-tolerated, high-flow nasal cannula (HFNC) may be suboptimal for more severe cases of respiratory distress syndrome (RDS) in preterm infants (14, 16).
- Nasal Trauma: Nasal skin breakdown remains a significant complication, particularly with prolonged nCPAP use. Preventive measures include hydrocolloid barriers, alternating interfaces, and vigilant skin checks (19).
- Protocol Variability: A lack of standardized protocols for the initiation, escalation, and weaning of NIV persists across institutions, highlighting the need for consensus guidelines and further research (22).
References
1. Greenough, A., Alberti, P., & Ade-Ajayi, N. (2025). Respiratory support strategies for surgical neonates: A review. Children, 12(6), Article e1009. https://pmc.ncbi.nlm.nih.gov/articles/PMC11941308/
2. Ako, A. A., Ismaiel, A., & Rastogi, S. (2025). Electrical impedance tomography in neonates: A review. Pediatric Research. https://www.nature.com/articles/s41390-025-03929-x
3. Hsu, J. F., Lin, Y. C., Lin, C. Y., Chu, S. M., & Cheng, H. J. (2025). Deep learning models for early and accurate diagnosis of ventilator-associated pneumonia in mechanically ventilated neonates. Computers in Biology and Medicine, 162, Article 107511. https://www.sciencedirect.com/science/article/pii/S0010482525002938
4. Chakkarapani, A. A., Adappa, R., Ali, S. K. M., & Gupta, S. (2020). Current concepts in assisted mechanical ventilation in the neonate: Part 2. International Journal of Pediatrics and Adolescent Medicine, 7(4), 179–186. https://doi.org/10.1016/j.ijpam.2020.07.006
5. Schulzke SM, Stoecklin B. Update on ventilatory management of extremely preterm infants. Pediatr Anesth. 2022;32(5):432-40. https://doi.org/10.1111/pan.14369
6. Keszler, M. (2017). Volume-targeted ventilation: One size does not fit all. Seminars in Fetal and Neonatal Medicine, 22(6), 369–375. https://doi.org/10.1016/j.siny.2017.08.002
7. Tingay, D. G., Dahm, S. I., & Sett, A. (2025). Are we ready for volume targeting during high-frequency oscillatory ventilation in neonates? Pediatric Research. https://www.nature.com/articles/s41390-025-04015-y
8. Hodgson, C. L., Tuxen, D. V., & Adritsos, D. (2020). Application of new ARDS guidelines at the bedside. Critical Care, 24(1), 1307-16. https://pmc.ncbi.nlm.nih.gov/articles/PMC6591785/
9. Van Kaam, A. H., & Rimensberger, P. C. (2007). Lung-protective ventilation strategies in neonatology: What do we know and what do we need to know? Critical Care Medicine, 35(3), 925–931. https://journals.lww.com/ccmjournal/Abstract/2007/03000/Lung_protective_ventilation_strategies_in.35.aspx
10. Shi, Y., & De Luca, D. (2019). Noninvasive respiratory support strategies after extubation in preterm neonates. BMC Pediatrics, 19, Article 1625. https://doi.org/10.1186/s12887-019-1625-1
11. Ozer, E. A. (2020). Lung-protective ventilation in neonatal intensive care unit. Journal of Clinical Neonatology, 9(3), 105–113. https://10.4103/jcn.JCN_96_19
12. Egbuta, C., & Easley, R. B. (2022). Update on ventilation management in the Pediatric Intensive Care Unit. Pediatric Anesthesia, 32(6), 698–708. https://doi.org/10.1111/pan.14374
13. Colaizy, T. T., Elgin, T. G., Berger, J. N., & Thomas, B. A. (2022). Ventilator management in extremely preterm infants. NeoReviews, 23(10), e661–e671. https://doi.org/10.1542/neo.23-10-e661
14. Shi, Y., Muniraman, H., & Biniwale, M. (2020). A review on non-invasive respiratory support for management of respiratory distress in extremely preterm infants. Frontiers in Pediatrics, 8, Article 270. https://doi.org/10.3389/fped.2020.00270
15. Yuan, G., Liu, H., Wu, Z., & Chen, X. (2021). Comparison of the efficacy and safety of three non-invasive ventilation methods in the initial treatment of premature infants with respiratory distress syndrome. International Journal of Clinical and Experimental Medicine, 14(2), 375–383. https://e-century.us/files/ijcem/14/2/ijcem0116814.pdf
16. More, K., Ramaswamy, V. V., & Roehr, C. C. (2020). Efficacy of noninvasive respiratory support modes for primary respiratory support in preterm neonates with respiratory distress syndrome: systematic review and meta-analysis. Pediatric Pulmonology, 55(6), 1325–1335. https://doi.org/10.1002/ppul.25011
17. Dassios, T., Kaltsogianni, O., & Greenough, A. (2023). Neonatal respiratory support strategies—short and long-term respiratory outcomes. Frontiers in Pediatrics, 11, Article 1212074. https://doi.org/10.3389/fped.2023.1212074
18. Karnati, S., & Sammour, I. (2020). Non-invasive respiratory support of the premature neonate: from physics to bench to practice. Frontiers in Pediatrics, 8, Article 214. https://doi.org/10.3389/fped.2020.00214
19. Boel, L., Hixson, T., Brown, L., Sage, J., & Kotecha, S. (2022). Non-invasive respiratory support in preterm infants. Paediatric Respiratory Reviews, 44, 1–10. https://doi.org/10.1016/j.prrv.2022.01.004
20. Ramaswamy, V. V., Devi, R., & Kumar, G. (2023). Non-invasive ventilation in neonates: a review of current literature. Frontiers in Pediatrics, 11, Article 1248836. https://doi.org/10.3389/fped.2023.1248836
21. Permall, D. L., Pasha, A. B., & Chen, X. (2019). Current insights in non-invasive ventilation for the treatment of neonatal respiratory disease. Italian Journal of Pediatrics, 45, 70. https://doi.org/10.1186/s13052-019-0707-x
22. Hussain, W. A., & Marks, J. D. (2019). Approaches to noninvasive respiratory support in preterm infants: from CPAP to NAVA. NeoReviews, 20(4), e213–e225.

