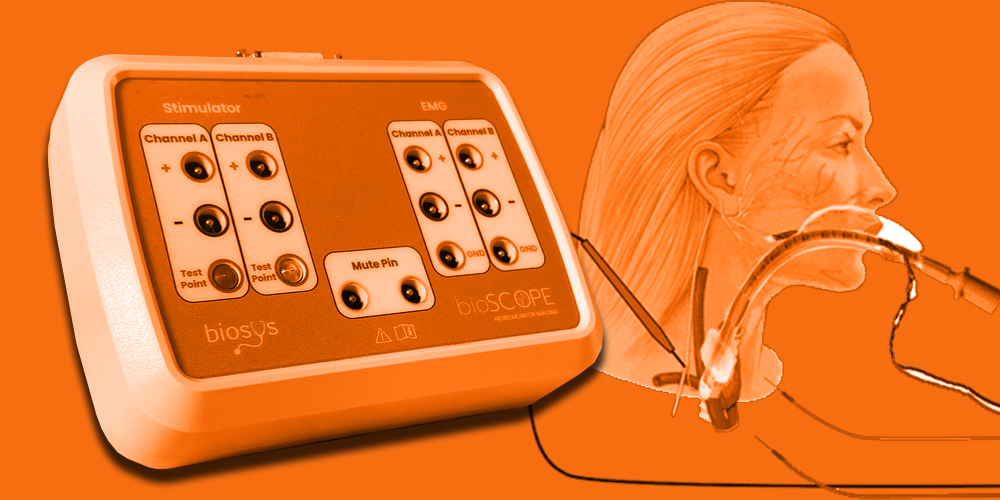
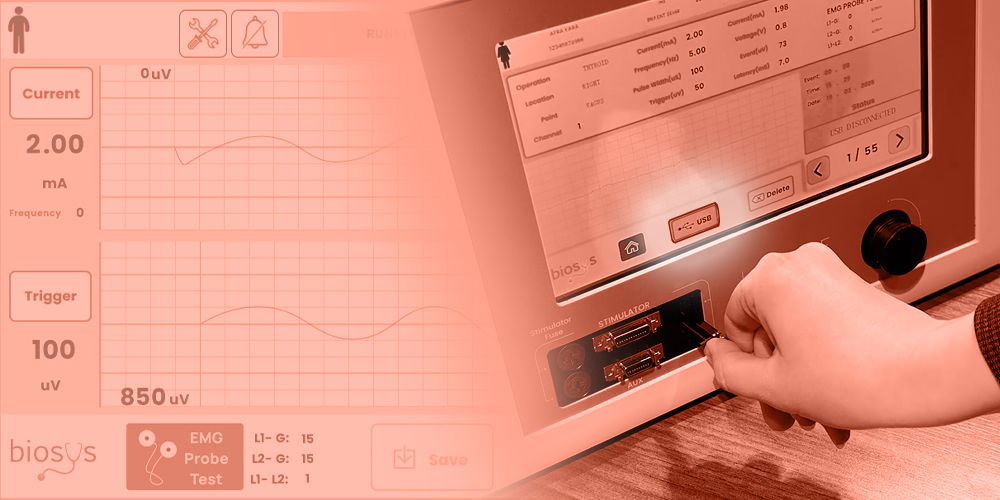
Neuromonitoring is a vital medical technology that provides real-time assessments of the nervous system’s functional integrity during surgeries and critical care situations. It plays a crucial role in preventing neurological damage, particularly in neurosurgery, spinal surgery, ENT procedures, and intensive care units (ICUs).
This technology, which includes techniques such as electroencephalography (EEG), electromyography (EMG), and evoked potentials, has evolved significantly since its inception in the early 20th century. The integration of these systems into clinical practice began gaining momentum in the 1970s and 1980s, improving surgical outcomes by enabling early detection of potential neural compromise.
The significance of neuromonitoring lies in its ability to enhance patient safety, reduce surgical risks, and optimize clinical decision-making by offering real-time neurophysiological feedback.
Furthermore, with advances in artificial intelligence (AI), cloud computing, and miniaturization, the future of neuromonitoring promises to enhance its precision, accessibility, and adaptability, further transforming its role in patient care.
This review explores the historical development, clinical applications, and future directions of neuromonitoring, highlighting its growing importance in modern medicine.
Introduction to Neuromonitoring
The origins of neuromonitoring date back to the early 20th century, with the invention of:
- Electroencephalography (EEG) in the 1920s by Hans Berger, which provided a method to monitor electrical activity in the brain (1).
- Electromyography (EMG) in the 1940s, allowing for the recording of muscle responses triggered by nerve stimulation (2).
However, intraoperative neuromonitoring (IONM) gained widespread adoption in the 1970s and 1980s, following advancements in signal processing, neurophysiology, and instrumentation that made real-time monitoring feasible during complex surgeries (3).
Neuromonitoring is fundamentally rooted in the principles of neurophysiology, the branch of physiology that explores the functional properties of the nervous system at cellular, molecular, and systemic levels (4).
The nervous system operates through the generation and propagation of electrical impulses known as action potentials, which are initiated by ion exchange across neuronal membranes (4). These action potentials are essential for transmitting information between neurons and from neurons to muscles or sensory receptors via synaptic transmission (4).
In clinical neuromonitoring, these electrical signals are captured and interpreted using a range of techniques that exploit the bioelectrical nature of the nervous system.
Electroencephalography (EEG) records spontaneous electrical activity of the cerebral cortex and is especially valuable in detecting cortical dysfunction or seizure activity (5).
Electromyography (EMG) measures the electrical activity produced by skeletal muscles and is widely used to assess peripheral nerve integrity, particularly during spinal and cranial surgeries (6).
Evoked potentials (EPs), such as Somatosensory Evoked Potentials (SSEPs) and Motor Evoked Potentials (MEPs), are used to monitor the functional pathways of the central nervous system by applying a stimulus and recording the response (7).
SSEPs test the integrity of ascending sensory pathways, while MEPs evaluate descending motor pathways, especially useful in spinal and neurosurgical procedures (7).
Advanced neuromonitoring often integrates multimodal approaches, combining several neurophysiological techniques simultaneously to provide comprehensive feedback on the functional status of different components of the nervous system (8).
These techniques rely on both surface electrodes (placed on the scalp or skin) and needle electrodes (inserted intramuscularly or subdermally) for precise localization and quantification of neural activity.
Clinically, these signals serve as real-time indicators of neural integrity. A sudden loss or attenuation of an evoked potential, for example, may indicate ischemia, mechanical compression, or direct trauma to a neural structure (9).
This real-time feedback allows surgeons and anesthesiologists to adjust surgical technique, reposition instruments, or modify anesthesia protocols to mitigate the risk of permanent neurological injury (9). The integration of neurophysiological principles into intraoperative and critical care monitoring has therefore become essential for enhancing surgical safety, improving neurological outcomes, and reducing medico-legal risks (9).
Neuromonitoring is a medical technology designed to assess and monitor the functional integrity of the nervous system in real-time during surgeries and critical care situations. It plays a vital role in neurosurgery, spinal surgery, ENT procedures, and critical care, enabling healthcare professionals to detect and prevent neurological damage before it becomes irreversible (9).
By continuously measuring electrical activity in the brain, spinal cord, and peripheral nerves, neuromonitoring enhances patient safety, surgical precision, and overall outcomes.

Definition of Neuromonitor
A neuromonitor, short for neurological monitor or neuromonitoring device, is a medical diagnostic tool designed to continuously assess and record electrical activity within the nervous system (10). It plays a critical role in detecting early signs of neurological impairment, especially during high-risk surgeries or in intensive care settings (11).
These devices are vital in ensuring the functional integrity of neural structures—such as the brain, spinal cord, and peripheral nerves—remains intact during procedures where these structures are vulnerable to injury (12).
Applications of Neuromonitors in Medicine
Neuromonitoring is utilized across a wide spectrum of clinical settings where real-time assessment of nervous system function is critical for patient safety. Its primary application is in the intraoperative environment, where it serves as a safeguard during surgeries that pose a risk to neural structures such as the brain, spinal cord, or peripheral nerves (2,8).
By continuously tracking neural signals, neuromonitoring helps surgeons detect early signs of nerve irritation or injury, enabling immediate corrective measures to prevent long-term deficits (3,8). Beyond the operating room, neuromonitoring is also employed in intensive care units (ICUs) to evaluate brain activity in patients with traumatic brain injury, stroke, or coma (11,13).
Additionally, it plays a key role in neurological diagnostics, such as epilepsy monitoring and assessing neuromuscular disorders (5,7). As such, neuromonitoring has become a cornerstone of modern neuroprotective strategies across various disciplines of medicine (9).
1. Intraoperative Neuromonitoring (IONM) in Surgery
Neuromonitors are extensively used during surgeries to monitor neural pathways and prevent neurological injuries. Their primary applications include:
- Neurosurgery: Used for procedures such as brain tumor removal, epilepsy surgery, and aneurysm clipping, where preserving functional brain areas is essential (5,8).
- Spinal Surgery: Ensures spinal cord integrity during procedures like scoliosis correction, spinal decompression, and spinal fusion surgeries using Somatosensory Evoked Potentials (SSEPs) and Motor Evoked Potentials (MEPs) (3,7,8).
- Peripheral Nerve Surgery: Protects motor and sensory nerves during surgeries involving limbs or facial nerve reconstruction (8).
- Vascular Surgery: Reduces the risk of stroke by monitoring brain activity and blood flow during carotid endarterectomy and aortic aneurysm repair (8).
Example: During spinal fusion surgery, SSEPs and MEPs are employed to continuously monitor the spinal cord’s functional integrity, minimizing the risk of postoperative neurological deficits (3,7).
2. Intensive Care Unit (ICU) & Neurocritical Monitoring
Neuromonitors are critical in the ICU for continuous assessment of brain function, particularly for patients with severe neurological conditions.
- Traumatic Brain Injury (TBI): Monitoring intracranial pressure (ICP) and cerebral perfusion (11,13).
- Stroke & Cerebral Ischemia: Evaluating blood flow and brain tissue oxygenation (13).
- Coma & Brain Death Assessment: Using Continuous EEG (cEEG) to detect non-convulsive seizures or assess brain activity in unresponsive patients (11,13).
- Brain Oxygenation Monitoring (PbtO₂, NIRS): Evaluating hypoxia and ischemia risks (13).
Example: In stroke patients, Transcranial Doppler (TCD) ultrasound is used to monitor cerebral blood flow and ensure adequate perfusion (13).
3. Epilepsy Monitoring & Diagnosis
Neuromonitors are vital tools in the diagnosis and management of epilepsy, particularly for patients with drug-resistant epilepsy.
- Long-Term EEG Monitoring: Detects abnormal electrical activity and localizes seizure origins (5,7,14).
- Video-EEG Monitoring: Simultaneously records physical symptoms and electrical activity during seizures (14).
- Intracranial Depth Electrodes: Used for mapping seizure-prone areas in surgical candidates (14).
Example: Neuromonitoring helps identify the precise brain region responsible for seizure activity, guiding surgical removal of the affected area when medication is ineffective (14).
4. Anesthesia & Sedation Monitoring
Neuromonitoring is increasingly used to optimize anesthesia and sedation levels during surgery.
- Bispectral Index (BIS) Monitoring: Measures depth of anesthesia to prevent under- or over-sedation (15).
- Processed EEG (pEEG): Adjusts anesthesia dosage based on real-time brain activity (15).
Example: BIS monitoring ensures patients receive the appropriate level of anesthesia during high-risk neurosurgical procedures, enhancing recovery and minimizing side effects (15).
5. Neurological Research & Brain-Computer Interfaces (BCI)
Neuromonitors are essential for developing brain-machine interfaces (BMIs) and enhancing neuroprosthetic technologies.
- Neuroprosthetics & Robotics: Allow paralyzed individuals to control external devices using brain signals (16).
- AI-Driven Cognitive Monitoring: Facilitates early detection of dementia and other neurodegenerative conditions (16).
- Neurofeedback therapy: Used in treating Attention-Deficit/Hyperactivity Disorder (ADHD), Post-Traumatic Stress Disorder (PTSD), and cognitive rehabilitation (16). Example: EEG-based BCIs allow individuals with severe disabilities to communicate and control assistive devices using brain signals alone (16).

Technical Aspects of Neuromonitors
Neuromonitoring systems rely on a combination of sophisticated hardware and intelligent software to deliver real-time insights into neural function. From core sensors and signal processing techniques to intuitive interfaces and portable designs, each component plays a vital role in ensuring accurate, reliable, and clinically meaningful monitoring.
1. Core Sensors & Electrodes Used in Neuromonitoring
Neuromonitors employ various sensors to capture neural activity:
- EEG Electrodes (Scalp & Depth Electrodes): Record brain electrical activity.
- EMG Electrodes: Measure muscle activity to evaluate nerve integrity.
- SSEP & MEP Electrodes: Stimulate and monitor sensory and motor pathways.
- Near-Infrared Spectroscopy (NIRS): Non-invasively detects brain oxygenation.
- Intracranial Pressure (ICP) Sensors: Monitor brain swelling in TBI patients.
2. Signal Processing & Interpretation
- Amplification: Enhances weak neural signals for accurate interpretation.
- Filtering: Removes artifacts caused by muscle movement, eye blinks, and external noise.
- Machine Learning Algorithms: AI-based software identifies early-stage seizures, cerebral ischemia, and other anomalies.
Example: AI-enhanced EEG systems can predict seizures several hours before clinical symptoms appear, allowing for early interventions.
3. User Interface & Data Visualization
Modern neuromonitors offer intuitive interfaces that provide:
- Real-time multi-channel waveforms.
- Automated alerts for abnormal neural activity.
- 3D Brain Mapping for surgical guidance.
- Remote Monitoring via cloud-based AI.
Example: Portable neuromonitors like the Natus Brain Monitor provide continuous EEG tracking in ICUs, with instant alerts sent to clinicians’ mobile devices.
4. Wireless & Portable Neuromonitoring Devices
Technological advancements have led to portable and wireless systems that enhance patient accessibility:
- Wearable EEG Headsets: Used for epilepsy monitoring and sleep disorder diagnosis.
- Telemedicine-Enabled Diagnostics: Allows remote monitoring and care.
- Wireless Neurostimulators: Assist patients with Parkinson’s disease and chronic pain.
Example: Bioscope by Biosys, initially a dual-channel neuromonitoring system for ENT, hand, and facial surgeries, is currently advancing to a 16-channel platform to expand its capabilities in both traditional and remote surgical environments.
Significance of Neuromonitoring
The nervous system, comprising the brain, spinal cord, and peripheral nerves, is highly vulnerable to injury during surgical interventions, trauma, or critical illness (4).
Even minimal damage to these structures can lead to devastating and often irreversible consequences such as motor deficits, sensory loss, chronic pain, paralysis, or cognitive dysfunction (4,9). This is particularly true during procedures involving delicate anatomical regions such as the spine, brainstem, cranial nerves, or peripheral nerve plexuses (8).
Intraoperative neuromonitoring (IONM) serves as a real-time surveillance system, enabling surgeons and anesthesiologists to continuously evaluate the functional integrity of neural pathways during surgical procedures (3,8).
By detecting subtle changes in neural activity, IONM provides early warning signs of potential nerve compromise before structural damage becomes permanent (8). This allows the surgical team to immediately adjust their technique—such as repositioning surgical tools, reducing traction, or altering the depth of anesthesia—to prevent injury (3,8).
Furthermore, neuromonitoring contributes to improved functional and neurological outcomes. Numerous clinical studies have shown that the use of IONM in spine and brain surgeries significantly reduces the incidence of postoperative neurological deficits (9,17).
For instance, in scoliosis correction or tumor resection surgeries, monitoring somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs) helps ensure spinal cord integrity throughout the operation (7,9,17).
In critical care settings, such as the intensive care unit (ICU), neuromonitoring technologies like EEG and bispectral index (BIS) allow clinicians to assess brain function in patients with coma, traumatic brain injury, or under sedation (11,13,15).
This enables timely interventions that may mitigate secondary brain injury, optimize ventilator management, or evaluate seizure activity (11,13,15).
In summary, the importance of neuromonitoring lies in its ability to:
- Enhance surgical safety by reducing the risk of intraoperative nerve damage (8,9).
- Support clinical decision-making with real-time neurophysiological feedback (8,9).
- Improve prognosis and long-term outcomes by preserving neurological function (9,17).
- Reduce healthcare burden by preventing complications that may lead to prolonged hospitalization, rehabilitation, or lifelong disability (17).
- Improves Surgical Precision: Guides surgeons to avoid critical nerve pathways (8,9).
- Reduces Post-Surgical Complications: Minimizes risks of paralysis and cognitive impairment (9,17).
- Supports Predictive Medicine: AI-enhanced systems enable early diagnosis of neurodegenerative diseases (16).
- Enhances Patient Recovery: Facilitates faster rehabilitation (9,17).
As surgical procedures become more complex and patient populations more vulnerable, the role of neuromonitoring becomes increasingly essential in delivering high-quality, patient-centered care (9,17).

Future of Neuromonitoring
Neuromonitoring is undergoing a transformative evolution driven by technological advancements, with the future promising smarter, more adaptive, and more accessible monitoring solutions (16,18). As surgical procedures grow increasingly complex and precision becomes paramount, the demand for advanced neuromonitoring capabilities has never been greater (9,18).
Future directions in this field are largely shaped by the integration of artificial intelligence (AI), machine learning, cloud-based platforms, and real-time remote monitoring, all of which aim to enhance safety, efficiency, and patient outcomes (16,18,19).
1. Artificial Intelligence and Predictive Analytics
AI is beginning to revolutionize how neuromonitoring data is interpreted. By applying machine learning algorithms to large datasets of neurophysiological signals, systems can identify subtle patterns and deviations that may precede neurological injury (16,18). This allows for predictive diagnostics, enabling clinicians to act before critical thresholds are crossed. For example, AI-enhanced systems can detect early signs of ischemia or nerve traction injuries based on changes in signal amplitude or latency long before these would be apparent to the human eye (16,18,19).
2. Cloud-Based Data Storage and Analysis
The integration of cloud computing facilitates secure, centralized storage and analysis of neuromonitoring data (20). This approach offers several benefits, including:
- Long-term tracking of patient neurological trends (20).
- Facilitating multi-center collaborations and comparative studies (20).
- Providing remote access to data for expert consultations, even during surgery (20).
- Enabling real-time audits and quality assurance for improved accountability and outcomes (20).
3. Remote and Telemonitoring Capabilities
With the rise of telemedicine, neuromonitoring systems are beginning to support remote supervision (21). This allows expert neurophysiologists to oversee and interpret intraoperative neuromonitoring from different locations, enhancing the availability of specialized care in rural or underserved hospitals (21). This development also supports the scalability of neuromonitoring services, ensuring that high-risk procedures can be safely conducted even in lower-resourced settings (21).
4. Miniaturization and Wearable Neuromonitors
The development of portable and wearable neuromonitoring devices is another key innovation on the horizon (22). These compact systems can continuously monitor neural activity outside the operating room — such as in ICU patients, during sleep studies, or in home rehabilitation settings — offering clinicians new ways to manage and follow neurological function over time (22).
5. Enhanced User Interfaces and Automation
Modern neuromonitoring systems are being designed with more intuitive user interfaces, automatic signal calibration, and smart artifact reduction features (19,22). These improvements reduce the cognitive and technical load on operating room staff and improve the accuracy and reliability of the recorded data (19,22).
6. Integration with Surgical Robotics and Navigation
As robot-assisted surgeries become more common, future neuromonitoring systems are being designed to integrate seamlessly with robotic platforms and navigation systems (23). This allows real-time neurophysiological feedback to directly guide robotic movement, reducing human error and increasing surgical precision (23).
The future of neuromonitoring is geared toward making surgeries safer, more efficient, and more patient-specific (18,19). With ongoing advances in AI, connectivity, hardware, and interface design, neuromonitoring is poised to become an even more integral part of precision medicine and surgical care (16,18,19).
These innovations will not only enhance intraoperative decision-making but also expand the reach of neuromonitoring into diagnostics, rehabilitation, and long-term neurological care (16,19,22).
References
- Berger, H. (1929). Über das Elektrenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten, 87(1), 527–570. https://doi.org/10.1007/BF01797193
- Preston, D. C., & Shapiro, B. E. (2013). Electromyography and neuromuscular disorders: Clinical-electrophysiologic correlations (3rd ed.). Elsevier.
- MacDonald, D. B. (2006). Intraoperative motor evoked potential monitoring: Overview and update. Journal of Clinical Monitoring and Computing, 20(5), 347–377. https://doi.org/10.1007/s10877-006-9040-6
- Purves, D., Augustine, G. J., & Fitzpatrick, D. (2018). Neuroscience (6th ed.). Oxford University Press.
- Niedermeyer, E., & da Silva, F. L. (2004). Electroencephalography: Basic principles, clinical applications, and related fields (5th ed.). Lippincott Williams & Wilkins.
- Dumitru, D., Amato, A. A., & Zwarts, M. J. (2002). Electrodiagnostic medicine (2nd ed.). Hanley & Belfus.
- Nuwer, M. R. (1998). Intraoperative monitoring of neural function. In Handbook of Clinical Neurophysiology (Vol. 3, pp. 841–871). Elsevier.
- Sala, F., & Deletis, V. (2007). Intraoperative neurophysiology in neurosurgery: A short overview. Clinical Neurophysiology, 118(3), 525–528. https://doi.org/10.1016/j.clinph.2006.10.021
- Skinner, S. A., Transfeldt, E. E., & Grafton, S. T. (2008). Intraoperative neurophysiologic monitoring: A review of techniques used to monitor the nervous system during spine surgery. Neurosurgical Focus, 25(3), E6. https://doi.org/10.3171/FOC/2008/25/9/E6
- MacDonald, D. B., Skinner, S., Shils, J., & Yingling, C. (2013). Intraoperative motor evoked potential monitoring – A position statement by the American Society of Neurophysiological Monitoring. Clinical Neurophysiology, 124(12), 2291–2316. https://doi.org/10.1016/j.clinph.2013.07.025
- Oddo, M., Bösel, J., Helbok, R., et al. (2018). Monitoring the injured brain: ICP and advanced neuromonitoring. Intensive Care Medicine, 44(12), 1888–1890. https://doi.org/10.1007/s00134-018-5448-2
- Nuwer, M. R., Dawson, E. G., Carlson, L. G., & Kanim, L. E. (1995). Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: Results of a large multicenter survey. Electroencephalography and Clinical Neurophysiology, 96(1), 6–11. https://doi.org/10.1016/0013-4694(95)00035-6
- Smith, S. J. M. (2005). EEG in the diagnosis, classification, and management of patients with epilepsy. Journal of Neurology, Neurosurgery & Psychiatry, 76(suppl 2), ii2–ii7. https://doi.org/10.1136/jnnp.2005.069245
- Rampil, I. J. (1998). A primer for EEG signal processing in anesthesia. Anesthesiology, 89(4), 980–1002. https://doi.org/10.1097/00000542-199810000-00023
- Lebedev, M. A., & Nicolelis, M. A. L. (2006). Brain–machine interfaces: past, present and future. Trends in Neurosciences, 29(9), 536–546. https://doi.org/10.1016/j.tins.2006.07.004
- Chuang, C. F., Chen, Y. J., Chang, Y. J., et al. (2021). Artificial intelligence applications in intraoperative neuromonitoring: A review. Frontiers in Medicine, 8, 655105. https://doi.org/10.3389/fmed.2021.655105
- Fehlings, M. G., Brodke, D. S., Norvell, D. C., & Dettori, J. R. (2010). The evidence for intraoperative neurophysiological monitoring in spine surgery: Does it make a difference? Spine, 35(9 Suppl), S37–S46. https://doi.org/10.1097/BRS.0b013e3181d82c74
- Varatharajan, R., & Navaneethakrishnan, S. (2020). Artificial intelligence based neural monitoring and robotics in surgery. Surgical Innovation, 27(6), 663–673. https://doi.org/10.1177/1553350620943535
- El-Osta, A., Elghazaly, A., & Said, M. (2022). Cloud computing in healthcare: Review and research challenges. Healthcare Technology Letters, 9(2), 45–53. https://doi.org/10.1049/htl2.12016
- Garcia, R. M., & Sherman, E. M. (2020). Tele-neuromonitoring: Intraoperative neurophysiological monitoring from a distance. Clinical Neurophysiology, 131(1), 305–312. https://doi.org/10.1016/j.clinph.2019.10.022
- Khan, M. J., Hong, M. J., & Hong, K. S. (2018). Decoding of four movement directions using hybrid NIRS-EEG brain-computer interface. Frontiers in Human Neuroscience, 12, 244. https://doi.org/10.3389/fnhum.2018.00244
- Baek, H., Chung, J., Kim, Y. J., & Park, Y. S. (2021). Real-time neurophysiological monitoring during robot-assisted neurosurgery. Frontiers in Surgery, 8, 658776. https://doi.org/10.3389/fsurg.2021.658776